Quality Assurance and Regulatory Affairs
Medical Devices/Pharmaceutical
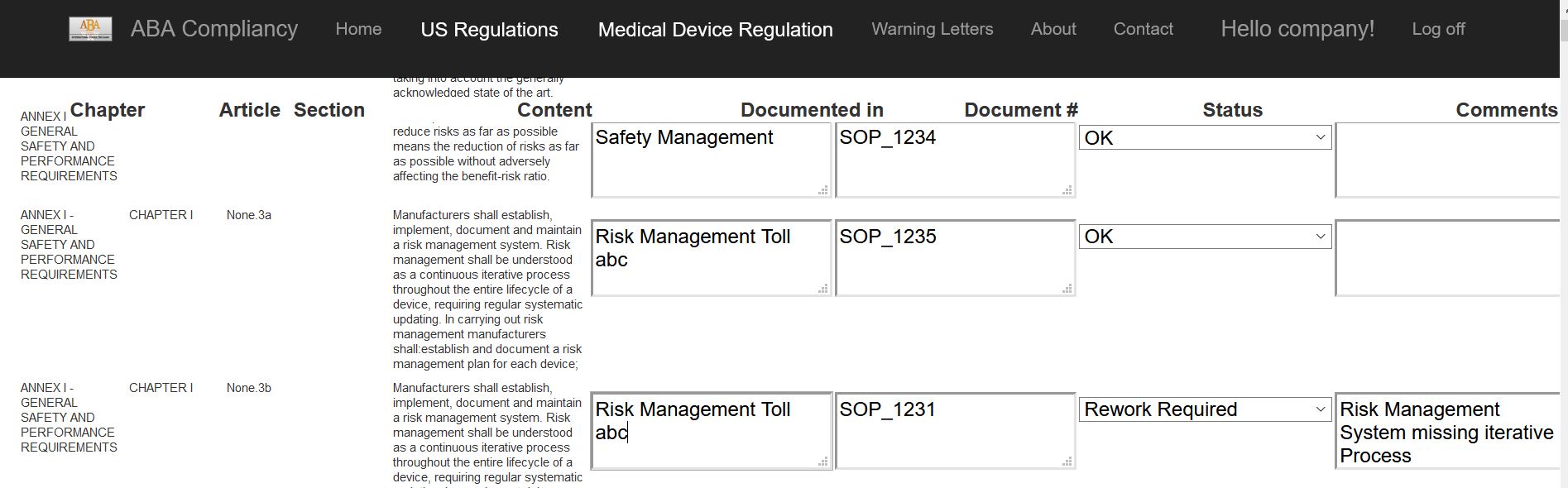
Standards, Guidelines and RegulationsWe help you to get your documents, applications and processes aligned with the appropriate norms, gudelines and regulations.
Do you need to make your documents, applications and processes conform to MDR, 21CFR820, ISO13485, IEC60601 or others?
We are happy to do it for you or assist your staff.
To do it in the most efficient way we are also using our own tools and checklists.
Our service also includes process- equipment- and computer system validations. All documents and data are created according to 21CFR11 and cGxP.
Remediation
We are experienced in international and cross-functional remediation projects for global players too. Here we can assist you in optimizing your quality system as well as in elaboration and submission of technical files
